Corrective action and preventive action pdf
Preventive or Corrective Actions are things that are done to ensure everyone’s safety, by looking into the possible risks and taking all the necessary precautions to prevent them.
[Company Name] Corrective and Preventive Action [Company Group, Division, Location] [Document Number] Rev x.xx DD/MM/YY [Document Filename] COMPANY PROPRIETARY AND …
Corrective Action is a reactive process to address concerns or issues after they have occurred. It assumes that a non-conformance or problem has been identified and has been reported by employees of the organisation or by customers or other interested parties / stakeholders. (more on Corrective Action) Preventive action. Preventive Action is a proactive process and is initiated to stop a
Root cause analysis and corrective action is a process for: finding the true cause(s) of events identifying and implementing corrective actions
Preventive action: action to eliminate the cause of a potential nonconformity or other potential undesirable situation. Corrective action: action to eliminate the cause of a nonconformity and to prevent recurrence.
The proposed corrective and preventive actions shall be verified during CAPA evaluation in form. 5.2 The “CAPA” form shall be treated as a tracking form of Corrective and Preventive actions from the source document.
A corrective and preventive action plan (CAPA) will identify the source of a problem and take corrective measures to avoid recurrences. When a problem has been solved, it will be added to the corrective action plan. A corrective and preventive action plan clarifies information about standards, protocols, procedures, and ongoing compliance. A corrective action report uses action items to …
How to Create a Corrective and Preventive Action Plan (CAPA) A CAPA is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document that the corrective action has resolved the problem. In general, the tone of CAPA should be
GUIDELINES FOR WRITING A CAPA Version June 2018 CAPA Template ORIA How to Create a Corrective and Preventive Action Plan (CAPA) A . CAPA . is written to identify a discrepancy or problem in the conduct of the clinical
Download PDF. Recommend Documents. CORRECTIVE AND PREVENTIVE ACTION – EUROLAB . EUROLAB ”Cook Book” – Doc No. 16.0 June 2013 1 CORRECTIVE AND PREVENTIVE ACTION Background of terms Corrective and preventive actions are powerful tools of. Corrective Action Request (CAR – Lockheed Martin . 7 The Basics: What is a CAR? A Corrective Action Request …
Skill level: Basic. Description. Capturing details regarding both corrective (fixing a problem that has occurred) and preventive (fixing a problem that could occur) actions can be a powerful tool for many business improvement opportunities.
CORRECTIVE AND PREVENTIVE ACTION. Definitions: (Quality Procedure D08) Corrective Action – action taken to eliminate the cause of an existing nonconformity, noncompliance, defect or other undesirable situation in order to prevent reoccurrence.
25/11/2014 · The purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and
The primary objective behind corrective action and preventive action (CAPA) in any pharmaceutical or medical device industry is to determine the weakness, deviation or failures and to carry out
Corrective and Preventive Action Agenda SAI Global
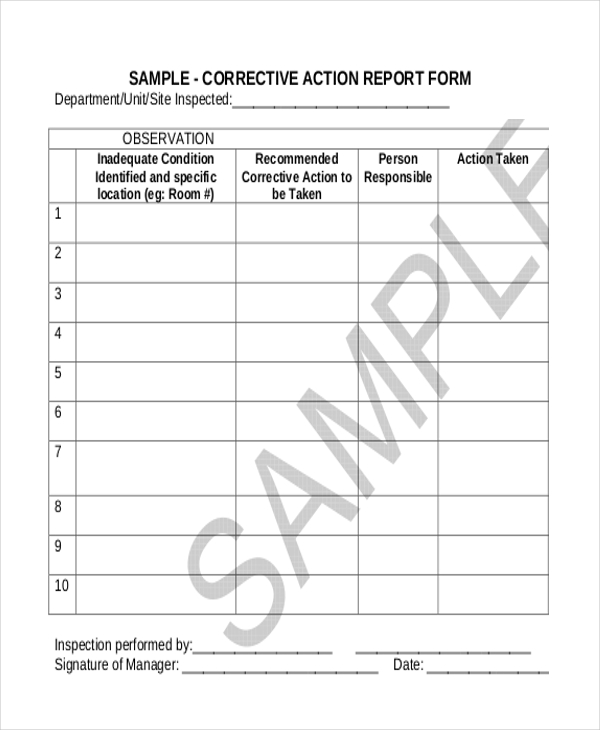
CORRECTIVE ACTION AND PREVENTIVE ACTION PLAN
4.1.3 A process to prioritize corrective or preventive actions based on the significance of risk 4.1.4 Setting deadlines for completion 4.1.5 A process to track corrective actions to closure and communicate status to leadership and affected personnel 4.1.6 A quality assurance process to confirm that corrective or preventive actions are adequate and appropriate to address the non-conformance …
Corrective & Preventive Action (CAPA) Software Detect, correct and manage compliance events seamlessly. At the heart of any Quality, EHS, or GMP Compliance management system, Corrective Action is a critical component to resolving adverse events.
SOP for Corrective Action and Preventive Action (CAPA) _ Pharmaceutical Guidelines – Download as PDF File (.pdf), Text File (.txt) or read online.
On the other hand, a corrective action report contains the discussion of the results of executing corrective actions in every circumstances or instances. Just like an affirmative action plan , it is highly recommended for you to develop a corrective action report in an objective and accurate manner.
EUROLAB ”Cook Book” – Doc No. 16.0 CORRECTIVE AND PREVENTIVE ACTION Background of terms Corrective and preventive actions are powerful tools of continuous improvement in quality management systems such as ISO 17025 and ISO 9001.
Corrective and preventative actions are regularly monitored and reviewed to ensure that what was decided has been implemented, and what was implemented has been effective. If you are in a meeting where monitoring and review is taking place, your job is to provide constructive
Buy Corrective/Preventive Action Procedures & Forms A critical requirement of ISO 9001 is corrective & preventive action (Sec. 8.5.2-8.5.3), and we are often asked the difference. Here is a quick evaluation and example to help you:
Guidance on corrective action and preventive action and related QMS processes GHTF/SG3/N18:2010 November 4, 2010 Page 3 of 26 Preface The document herein was produced by the Global Harmonization Task Force, a voluntary group

HS309 Corrective Action Procedure Page 3 of 4 Version: 4.3 Effective 24 February 2016 . 3.4 Review and monitoring of corrective action • Corrective actions are reviewed and monitored by school or unit H&S committee (Level 3) by monitoring the corrective action status report. All new hazards should be documented in the school or unit hazard and risk register. • Faculty and Divisional H&S
corrective/preventive action for effectiveness prior to its closure. 2.2 Senior Management The Senior Management, as needed, assists with determining the corrective action, taking into consideration root causes and preventive actions that need to be taken, and reviews the completed corrective/preventive action for effectiveness prior to its closure. 2.3 Facility Manager The Facility …
25/10/2017 · A Corrective Action Plan is a plan constructed to describe the course of action to be taken to resolve a CAR in the CAR/PAR System.€ A CAP is required of CARs expected to take more than 30 calendar days to resolve.€
Quality professionals frequently express confusion as to the difference between corrective and preventive action. A corrective action deals with a nonconformity that has occurred, and a preventive action addresses the potential for a nonconformity to occur. Many ISO 9000 registrar auditors tell
Team approach to corrective and preventive action . Step 1: Define the Problem • Introduction to the “7-D Process” approach and the “5 Why’s” • Writing a problem statement • Workshop I: Define the Problem . Step 2: Isolate and Contain the Symptom • Basic steps for isolating and containing the symptom • Workshop 2: Isolate and Contain the Symptom . Step 3: Determine the Root
Corrective_and_Preventive_Actions_V2.doc A corrective action procedure is started after a complaint or feedback by a customer, or upon detection of a nonconformity (e.g. during a quality audit).
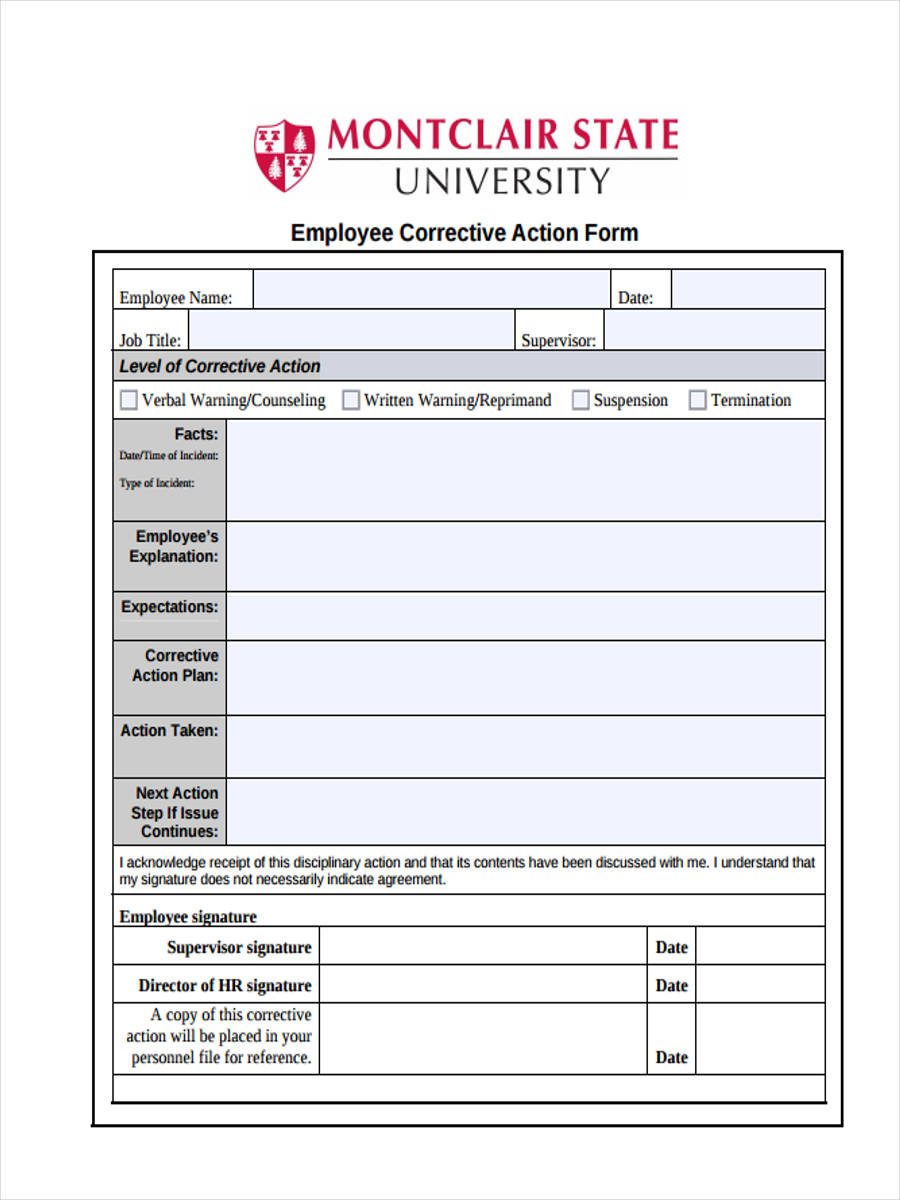
Verse Solutions Corrective and Preventive Action software (CAPA) features the ability to run multiple types of workflows, each with its own unique process. At the heart of any Quality, EHS, or GMP Compliance management system, Corrective Action is a critical component to …
2.2 A non-conformance is identified from the process Q2-830-01 or from an audit (Q2-822-01) as needing a CPAR. It is sent to the process owner and/or manager.
Preventive Action and Quality Improvement Preventive_Action(017)_AF.R3 Effective Date: June 20, 2013 1.4.2 Corrective Action An action initiated in response to an identified nonconformance, in order to define a problem, address a problem and attempt to identify the root cause of a problem. 1.4.3 Complaint A written or verbal notification received from within SESD or from an individual or
4.1.1 When implementing corrective or preventive action, the amount of time and effort will take into account the significance of the problem. The potential impact on the product, the development process, and the user will be evaluated. 4.1.2 Sources of information for corrective and preventive action will include customer complaints, technical support records, reports from training and
Corrective actions, on the other hand, provide managers with not only the data they need to construct an effective and efficient corrective action process, but can be used as input into preventive actions.
CORRECTIVE ACTION AND PREVENTIVE ACTION PLAN Date Effective: 12 April 2016 Form No. QWP-SL-RFO-1-05 Annex 1 Rev 02 Authorized by: RFO-NCR, Records Management Team Corrective Action and Preventive Action Plan Page 1 of 3
procedures for implementing corrective and preventive action, as required by 21 CFR 820.100(a) and (b). For-example: • (a) The procedure titled corrective Action Handling [redacted] was not approved and implemented to address corrective and preventive action and no established procedure was found to have been in place. Tonya White-Salters Identify Implement Review Verify Analyze. Tonya White
Details about the issue are recorded on the Corrective/Preventive Action Request form. 5.1.6. The Quality Assurance Project Manager will assign an investigator and schedule based on the degree of the potential non-conformance. 5.2. Investigating a request for Preventive Action 5.2.1. The investigator determines the cause of the potential non-conformance and designs the implementation plan to
CAPA and Risk Management “FDA agrees that the degree of corrective and preventive action taken to eliminate or minimize actual or potential nonconformities must be appropriate to the magnitude of
4 The process used for corrective actions and preventive actions is very similar and the steps outlined in this document can be used for either.
Corrective_and_Preventive_ACTION Production And
Root Cause & Corrective Action (RCCA) Overview 1 . Page Objective To provide guidance to carry out proper Root Cause Analysis (RCA) with suitable quality tools To ensure responded SCAR able to meet Keysight expectation Keysight Restricted 2 . Page Introduction Supplier Corrective Action Request (SCAR) is a systematic approach to request investigation of a problem that already happened and
Division) engages in corrective and preventive action to discover, investigate, and correct nonconformance’s related to F&S products, its processes, and the Division’s quality system. 1.2.
A preventive action is a change implemented to address a weakness in a management system that is not yet responsible for causing nonconforming product or service. Candidates for preventive action generally result from suggestions from customers or participants in the process but preventive action is a proactive process to identify opportunities for improvement rather than a simple reaction to
The purpose of this procedure is to have a defined method in applying preventive actions to eliminate the cause of potential non-conformities on the established …
Corrective Action Preventive Action (CAPA) Report Automated systems make corrective action preventive action (CAPA) reports a breeze for companies A CAPA report is a mechanism for correcting and recording defects and nonconformances.
To perform the root cause analysis, select and implement best action To monitor and evaluate the implementation of the selected corrective (preventive) action To validate the effectiveness of the implementation To report to the Unit Head on the effectiveness of the implementation To collect and send all records to the Quality Manager upon successfully ending the process.
The corrective preventive action module is designed with a configurable workflow that guides the CAPA through the process, which is defined and configured to meet your organizations unique needs. From root cause analysis, to the development of action plans, to verification of effectiveness – the corrective action module is designed to provide complete traceability of the data within the
Processing Corrective Actions/Preventive Actions Use. You use corrective and/or preventive actions to intervene in processes that were found to have potential for improvement during the audit.
EUROLAB ”Cook Book” – Doc No. 16.0 June 2013 1 CORRECTIVE AND PREVENTIVE ACTION Background of terms Corrective and preventive actions are powerful tools of continuous improvement in quality management
Ombu Enterprises, LLC Corrective & Preventive Action Warning Letter Page 1 of 2 . Corrective and Preventive Action . The Context . Device manufacturers encounter problems (actual nonconformances) which they investigate to – corporate fraud handbook prevention and detection 5th edition pdf A preventive action is a process to eliminate the cause of a potential nonconformity or other undesirable situation. – There can be more than one cause for a potential non-
The action was taken to rectify, fix or correct a specific deviation, defect or undesirable situation. Preventive Action The action was taken to eliminate the cause of deviation, defect, or other undesirable situation in order to prevent the future occurrence of such or similar an event.
Corrective and preventive action LIMSWiki
Corrective & Preventive Action (CAPA) Software Create
Corrective and Preventive Action Training PDF documents
Corrective Action Preventive Action Report MasterControl

Corrective Action and Preventive Action (CAPA
A review on corrective action and preventive action (CAPA
Corrective vs. Preventive Action ASQ
Corrective and Preventive Actions – ASQ Service Quality
–

Corrective Action Preventive Action Report MasterControl
Corrective & Preventive Action APB Consultant
4 The process used for corrective actions and preventive actions is very similar and the steps outlined in this document can be used for either.
The purpose of this procedure is to have a defined method in applying preventive actions to eliminate the cause of potential non-conformities on the established …
A corrective and preventive action plan (CAPA) will identify the source of a problem and take corrective measures to avoid recurrences. When a problem has been solved, it will be added to the corrective action plan. A corrective and preventive action plan clarifies information about standards, protocols, procedures, and ongoing compliance. A corrective action report uses action items to …
How to Create a Corrective and Preventive Action Plan (CAPA) A CAPA is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document that the corrective action has resolved the problem. In general, the tone of CAPA should be
On the other hand, a corrective action report contains the discussion of the results of executing corrective actions in every circumstances or instances. Just like an affirmative action plan , it is highly recommended for you to develop a corrective action report in an objective and accurate manner.
Preventive Action and Quality Improvement Preventive_Action(017)_AF.R3 Effective Date: June 20, 2013 1.4.2 Corrective Action An action initiated in response to an identified nonconformance, in order to define a problem, address a problem and attempt to identify the root cause of a problem. 1.4.3 Complaint A written or verbal notification received from within SESD or from an individual or



